STIOLTO RESPIMAT offers

*STIOLTO RESPIMAT is NOT a rescue medication and does NOT replace fast-acting inhalers to treat acute symptoms.
HELPS PREVENT EXACERBATIONS
STIOLTO RESPIMAT — proven to help prevent exacerbations1-3
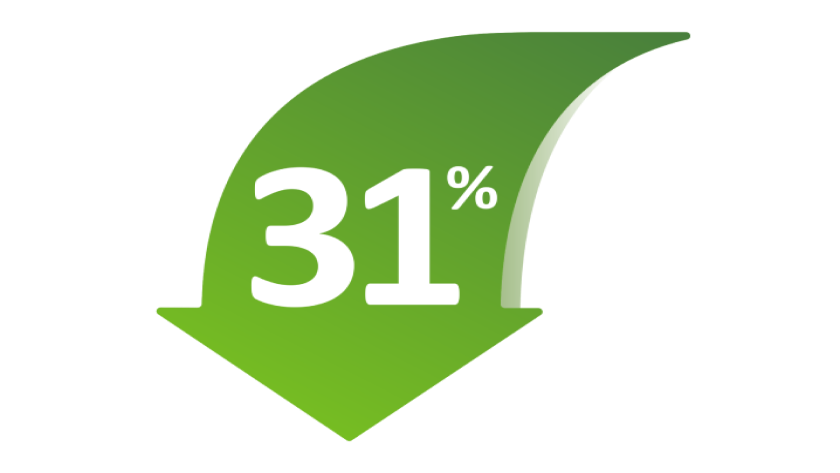
31% reduction in risk of exacerbations with Tiotropium compared with Placebo1-3
Tiotropium, an active ingredient of STIOLTO RESPIMAT, reduced the risk of exacerbations by 31% (RR=0.69 [95% Cl: 0.63, 0.77]; P<0.0001) and reduced the risk of exacerbation-related hospitalizations, independent of ICS use, by 27% vs placebo (RR=0.73 [95% CI: 0.589, 0.901]; P=0.0191).
ICS, inhaled corticosteroid.
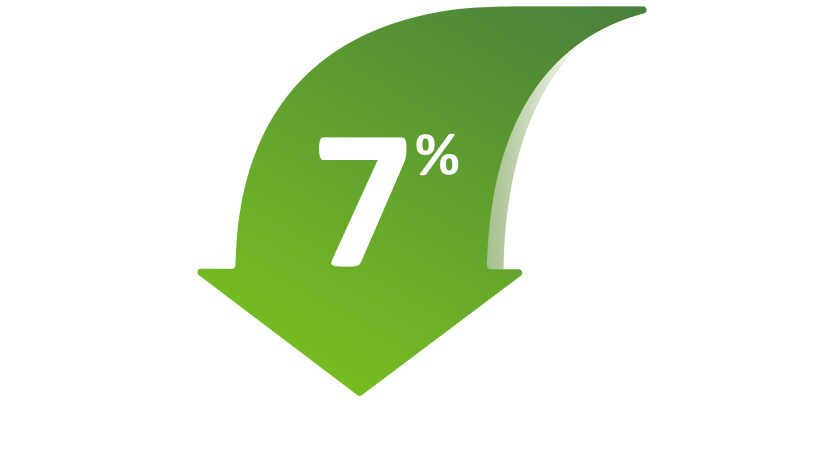
7% reduction in annualized rate of moderate and severe exacerbations compared with SPIRIVA RESPIMAT1,3
STIOLTO RESPIMAT reduced the annualized rate of moderate and severe exacerbations vs tiotropium bromide by 7% (RR=0.93 [99% Cl: 0.85, 1.02]; P=0.049). The P value of 0.049 did not meet the prespecified significance level of 0.01.
Study Designs
Expand allIn a 1-year, randomized, double-blind, parallel-group study, 3991 patients with COPD were evaluated to compare tiotropium via RESPIMAT and placebo (n=1939 tiotropium and n=1953 placebo were evaluable for efficacy) coprimary endpoints: change in trough FEV₁ from treatment Day 1 to Day 337, and time to first COPD exacerbation. Secondary endpoints were changes in trough FEV₁ at Days 29 and 169 and in trough FVC at Days 29, 169, and 337, the number of exacerbations per patient, the number of patients with ≥1 exacerbation, and the time to first exacerbation-related hospitalization. Exacerbations were defined as a complex of respiratory events or symptoms that lasted ≥3 days and required treatment with antibiotics and/or systemic corticosteroids, or prompted the investigator to change the patient's regular respiratory medication.2
The majority of patients in both treatment groups were on concomitant maintenance medication. All respiratory medications except inhaled anticholinergics were permitted.
Major inclusion criteria included patients with a diagnosis of COPD, 40 years of age or over, a prebronchodilator FEV₁ of ≤60% of predicted normal and a ratio of FEV₁ to FVC of ≤70%, and a smoking history of 10 pack-years or more.
FEV₁, forced expiratory volume in 1 second; FVC, forced vital capacity.
A 52-week, double-blind, parallel-group trial that included patients with COPD and a history of exacerbations who were randomized 1:1 using a randomized block design to receive STIOLTO RESPIMAT 5 mcg-5 mcg or SPIRIVA® RESPIMAT® (tiotropium bromide) Inhalation Spray 5 mcg once daily. The primary endpoint was the rate of moderate and severe COPD exacerbations, at a significance level of 0.01. A total of 7903 patients were randomized in the study and 7880 were treated; 3939 received STIOLTO RESPIMAT and 3941 received SPIRIVA RESPIMAT.3
-
§
In one 12-week trial (OTEMTO® 1), SGRQ responder rates at week 12 (defined as an improvement in score of 4 or more) were 53% (n=104/196), 42% (n=80/192), and 31% (n=58/186) for STIOLTO RESPIMAT, tiotropium bromide, and placebo, respectively, with odds ratios of 1.6 (95% CI: 1.1, 2.4) and 2.5 (95% CI: 1.6, 3.8) for STIOLTO RESPIMAT vs tiotropium bromide and STIOLTO RESPIMAT vs placebo, respectively. Similar results were confirmed in a replicate trial (OTEMTO® 2) and in two 52-week trials at Week 24 (TONADO® 1 and 2)
FAQs
Expand all- STIOLTO RESPIMAT — proven to help prevent exacerbations.1-3
- Tiotropium, an active ingredient of STIOLTO RESPIMAT, has been proven to help prevent COPD exacerbations, with a 31% reduction in risk of exacerbations with tiotropium compared with placebo plus a 27% reduction in risk of exacerbation-related hospitalizations - observed in clinical trials.1,2
Time without symptoms
Activity levels
Social impact
Note:
*In one 12-week trial (OTEMTO® 1), SGRQ responder rates at week 12 (defined as an improvement in score of 4 or more) were 53% (n=104/196), 42% (n=80/192), and 31% (n=58/186) for STIOLTO RESPIMAT, tiotropium bromide, and placebo, respectively, with odds ratios of 1.6 (95% CI: 1.1, 2.4) and 2.5 (95% CI: 1.6, 3.8) for STIOLTO RESPIMAT vs tiotropium bromide and STIOLTO RESPIMAT vs placebo, respectively. Similar results were confirmed in a replicate trial (OTEMTO® 2) and in two 52-week trials at Week 24 (TONADO® 1 and 2)
STIOLTO® RESPIMAT® (tiotropium bromide and olodaterol) Inhalation Spray is a combination of tiotropium, an anticholinergic, and olodaterol, a long-acting beta2-adrenergic agonist (LABA), indicated for the long-term, once-daily maintenance treatment of patients with chronic obstructive pulmonary disease (COPD), including chronic bronchitis and/or emphysema.
Important Limitations of Use
STIOLTO is NOT indicated to treat acute deterioration of COPD and is not indicated to treat asthma.
CONTRAINDICATION
Use of a LABA, including STIOLTO RESPIMAT, without an inhaled corticosteroid (ICS) is contraindicated in patients with asthma.
STIOLTO is contraindicated in patients with hypersensitivity to tiotropium, ipratropium (atropine derivatives), olodaterol, or any component of this product.
In clinical trials and postmarketing experience with tiotropium, immediate hypersensitivity reactions, including angioedema (including swelling of the lips, tongue, or throat), itching, or rash have been reported. Hypersensitivity reactions were also reported in clinical trials with STIOLTO.
WARNINGS AND PRECAUTIONS
LABA as monotherapy (without an ICS), for asthma increases the risk of asthma-related death, and in pediatric and adolescent patients, increases the risk of asthma-related hospitalizations.
Do not initiate STIOLTO in patients with acutely deteriorating COPD, which may be a life-threatening condition, or used as rescue therapy for acute symptoms. Acute symptoms should be treated with an inhaled short-acting beta2-agonist.
STIOLTO should not be used more often or at higher doses than recommended, or with other LABAs as an overdose may result.
If immediate hypersensitivity reactions occur, such as urticaria, angioedema, rash, bronchospasm, anaphylaxis, or itching, discontinue STIOLTO at once and consider alternative treatment. Patients with a history of hypersensitivity reactions to atropine or its derivatives should be closely monitored for similar hypersensitivity reactions to STIOLTO.
If paradoxical bronchospasm occurs, discontinue STIOLTO immediately and institute alternative therapy.
STIOLTO can produce a clinically significant cardiovascular effect in some patients, as measured by increases in pulse rate, systolic or diastolic blood pressure, and/or symptoms. If such effects occur, STIOLTO may need to be discontinued.
Use caution in patients with convulsive disorders, thyrotoxicosis, diabetes mellitus, ketoacidosis, in patients with known or suspected prolongation of the QT interval, and in patients who are unusually responsive to sympathomimetic amines.
Use with caution in patients with narrow-angle glaucoma. Instruct patients to contact a physician immediately if signs or symptoms of acute narrow-angle glaucoma develop.
Use with caution in patients with urinary retention especially in patients with prostatic hyperplasia or bladder-neck obstruction. Instruct patients to consult a physician immediately should any of these signs or symptoms develop.
Patients with moderate to severe renal impairment (creatinine clearance of <60 mL/min) should be monitored closely for anticholinergic side effects.
Be alert to hypokalemia and hyperglycemia.
ADVERSE REACTIONS
The most common adverse reactions with STIOLTO (>3% incidence and higher than an active control) were: nasopharyngitis, 12.4% (11.7%/12.6%), cough, 3.9% (4.4%/3.0%), and back pain, 3.6% (1.8%/3.4%).
DRUG INTERACTIONS
Use caution if administering adrenergic drugs because sympathetic effects of olodaterol may be potentiated.
Concomitant treatment with xanthine derivatives, steroids, or diuretics may potentiate any hypokalemic effect of olodaterol.
Use with caution in patients taking non–potassium-sparing diuretics, as the ECG changes and/or hypokalemia may worsen with concomitant beta-agonists.
The action of adrenergic agents on the cardiovascular system may be potentiated by monoamine oxidase inhibitors or tricyclic antidepressants or other drugs known to prolong the QTc interval. Therefore, STIOLTO should be used with extreme caution in patients being treated with these drugs. Use beta-blockers with caution as they not only block the therapeutic effects of beta-agonists, but may produce severe bronchospasm in patients with COPD.
Avoid co-administration of STIOLTO with other anticholinergic-containing drugs as this may lead to an increase in anticholinergic adverse effects.
STIOLTO is for oral inhalation only.
The STIOLTO cartridge is only intended for use with the STIOLTO RESPIMAT inhaler.
Inform patients not to spray STIOLTO into the eyes as this may cause blurring of vision and pupil dilation.
CL-STO-100021 6.5.2019
Please see full Prescribing Information, Patient Information, and Instructions for Use for STIOLTO RESPIMAT.
References
-
STIOLTO RESPIMAT [prescribing information]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc; January 2025.
-
Bateman ED, Tashkin D, Siafakas N, et al. A one-year trial of tiotropium RESPIMAT® plus usual therapy in COPD patients. Respir Med. 2010;104(10):1460-1472.
-
Calverley PMA, Anzueto AR, Carter K, et al. Tiotropium and olodaterol in the prevention of chronic obstructive pulmonary disease exacerbations (DYNAGITO): a double-blind, randomised, parallel-group, active-controlled trial. Lancet Respir Med. 2018;6(5):337-344.
-
Singh D, Ferguson GT, Bolitschek J, et al. Tiotropium + olodaterol shows clinically meaningful improvements in quality of life. Respir Med. 2015;109(10):1312-1319.
-
Jones PW, Forde Y. St George's Respiratory Questionnaire Manual. London, UK: St George's, University of London; 2009.
-
Data on file. Boehringer Ingelheim Pharmaceuticals, Inc.



